Central American and Caribbean Consensus for the Treatment of Multiple Sclerosis and Therapeutic Attitudes Facing the COVID-19 Pandemic
Abstract
Therapeutic decisions for multiple sclerosis have become more complex in the Central American and Caribbean region (CAC), with new treatments appearing every year but with well-known limitations in terms of access and application. Concomitantly, the advent of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic, with an increasing number of cases in the region, has increased the need for a consensus on therapeutic decisions to be made for people with multiple sclerosis. Under these circumstances, a reference framework is needed to gather current information to assist neurologists in making therapeutic decisions.
An evidence-based consensus on the use of disease-modifying therapies for multiple sclerosis was proposed and accomplished by the Central American and Caribbean Multiple Sclerosis Forum (FOCEM), including recommendations for treatment during the pandemic. Using the consensus panel development methodology, after a bibliographic review of the best quality and actualized information, a final report was written; this includes statements that reached more than 70% consensus among the panel of experts.
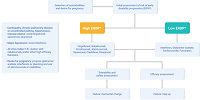
The recommendations encompass indications for drugs available for multiple sclerosis, definitions of therapeutic failure, patient follow-up, factors of poor prognosis, discontinuation of treatment, treatment during pregnancy and lactation and specific recommendations to apply during the SARS-CoV-2 pandemic.
Full text article
References
2. Gracia F, Armién B, Rivera V, et al. Multiple Sclerosis in Central American and Spanish Caribbean Region: Should it be Recognized as a Public Health Problem? Collaborative Multiple Sclerosis Group of Central America and Spanish Caribbean Region (CMSG). J Epid Prev Med. 2017;3:134.
3. Thompson AJ, Banwell BL, Barkhof F, et al. Position Paper Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162-173
4. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis. The 2013 revisions. Neurology. 2014;83:278-286
5. Abad Herrera P, Nogales Gaete J, Rivera V, et al. Documento de consenso de LACTRIMS para el tratamiento farmacológico de la esclerosis múltiple y sus variantes clínicas. Rev Neurol. 2012;55:737-748.
6. Waggoner J, Carline JD, Durning SJ. Is there a consensus on consensus methodology? Descriptions and recommendations for future consensus research. Acad Med. 2016;91:663–668.
7. Scolding N, Barnes D, Cader S, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015;15:273–279.
8. Kappos L, Li DKB, Stüve O, et al. Safety and Efficacy of Siponimod (BAF312) in Patients With Relapsing-Remitting Multiple Sclerosis: Dose-Blinded, Randomized Extension of the Phase 2 BOLD Study. JAMA Neurol. 2016;73:1089-1098.
9. Selmaj K, Li DK, Hartung H-P, et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol. 2013;12:756–67.
10. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med. 2017;376:209-220
11. Merck Pharmaceutical Companies. Mavenclad (Cladibrine) [package insert]. U.S. Food and Drug Administration website.www.accessdata.fda.gov/drugsatfda_docs/label/2019/022561s000lbl.pdf. Revised May 2020. Accessed 7 May, 2020.
12. Biogen Pharmaceutical Companies. Vumerity (diroxemil fumarate) [package insert]. U.S. Food and Drug Administration website.www.accessdata.fda.gov/drugsatfda_docs/label/2019/211855s000lbl.pdf. Revised May 2020. Accessed 7 May, 2020.
13. Bristol Meyers Squibb Pharmaceutical Companies. Zeposia (ozanimod) [package insert]. U.S. Food and Drug Administration website.www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf. Revised May 2020. Accessed 7 May, 2020.
14. Novartis Pharmaceutical Companies. Mayzent (siponimod) [package insert]. U.S. Food and Drug Administration website. www.accessdata.fda.gov/drugsatfda_docs/label/2019/209884s000lbl.pdf. Revised May 2020. Accessed 7 May, 2020.
15. Steinberg J, Fragoso YD, Duran Quiroz JC et al. Practical Issues Concerning the Approval and Use of Biosimilar Drugs for the Treatment of Multiple Sclerosis in Latin America. Neurol Ther. 2017;8:207-214
16. Correale J. Follow-on products for treatment of multiple sclerosis in Latin America: An update. J Neurol Sci. 2017;381:153–9.
17. Daams M, Steenwijk MD, Wattjes MP, et al. Unraveling the neuroimaging predictors for motor dysfunction in long-standing multiple sclerosis. Neurology. 2015;85:248-255
18. Fahrbach K, Huelin R, Martin AL, et al. Relating relapse and T2 lesion changes to disability progression in multiple sclerosis: a systematic literature review and regression analysis. BMC Neurol. 2013;13:180
19. Goodin D, Traboulsee A, Knappertz V, et al. Relationship between early clinical characteristicsand long term disability outcomes: 16 year cohortstudy (follow-up) of the pivotal interferon b-1b trial inmultiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83:282–287.
20. Hirst C, Ingram G, Swingler R, et al. Change in disability in patients with multiple sclerosis: a 20-year prospective population-based analysis. J Neurol Neurosurg Psychiatry. 2008;79:1137–1143.
21. Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of disability worsening in clinically isolated syndrome. Ann Clin Transl Neurol. 2015;2:479–91.
22. Maghzi AH, Revirajan N, Julian LJ, et al. Magnetic resonance imaging correlates of clinical outcomes in early multiple sclerosis. Mult Scler Relat Disord. 2014;3:720–727.
23. Minneboo A, Barkhof F, Polman CH, et al. Infratentorial Lesions Predict Long-term Disability in Patients With Initial Findings Suggestive of Multiple Sclerosis. Arch Neurol. 2004;61:217–21.
24. Tintore M, Rovira A, Arrambide G, et al. Brainstem lesions in clinically isolated syndromes. Neurology. 2010;75:1933-1938
25. Ruet A, Arrambide G, Brochet B, et al. Early predictors of multiple sclerosis after a typical clinically isolated syndrome. Mult Scler. 2014;20:1721-1726.
26. Tintore M, Rovira A, Río J, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain. 2015;138:1863-1874
27. Khan o, Williams MJ, Amezcua L, et al. Multiple sclerosis in US minority populations: Clinical practice insights. Neurol Clin Pract. 2015;5:132-142.
28. Río J, Castilló J, Rovira A, et al. Measures in the first year of therapy predict the response to interferon β in MS. Mult Scler. 2009;15:848–853.
29. Sormani M, Rio J, Tintorè M, et al. Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler. 2013;19:605–612.
30. Correale J, Abad P, Alvarenga R, et al. Management of relapsing–remitting multiple sclerosis in Latin America: Practical recommendations for treatment optimization. J Neurol Sci. 2014;339:196–206.
31. Río J, Rovira A, Tintoré M, et al. Disability progression markers over 6–12 years in interferon-β-treated multiple sclerosis patients. Mult Scler. 2018;24:322-330.
32. Lu G, Beadnall HN, Barton J, et al. The evolution of “No Evidence of Disease Activity” in multiple sclerosis. Mult Scler Relat Disord. 2018;20:231–238.
33. Stangel M, Penner I, Kallman B, et al. Towards the implementation of ‘no evidence of disease activity’ in multiple sclerosis treatment: the multiple sclerosis decision model. Ther Adv Neurol Disord. 2015;8:3–13.
34. Harding K, Williams O, Wills M, et al. Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients with Multiple Sclerosis. JAMA Neurol. 2019;76: 536-541.
35. Rosti-Otajärvi E, Ruutiainen J, Huhtala H, Hämäläinen P. Cognitive performance profile in different phenotypes of MS with cognitive complaints. Mult Scler Relat Disord. 2014;3:463-472
36. Cadavid D, Cohen J, Freedman MS, et al. The EDSS-Plus, an improved endpoint for disability progression in secondary progressive multiple sclerosis. Mult Scler. 2017;23:94-105.
37. Haghikia A, Langer-Gould A, Rellensmann G, et al. Natalizumab use during the third trimester of pregnancy. JAMA Neurol. 2014;71:891–895.
38 Ebrahimi N, Herbstritt S, Gold R, et al. Pregnancy and fetal outcomes following natalizumab exposure in pregnancy. A prospective, controlled observational study. Mult Scler. 2015;21:198–205.
39. Bove R, Alwan S, Friedman J, et al. Management of MS during pregnancy and reproductive years. Obstet Gynecol. 2014;124:1157–68.
40. Coyle PK. Management of women with multiple sclerosis through pregnancy and after childbirth. Ther Adv Neurol Disord. 2016;9:198–210.
41. Lu E, Wang BW, Guimond C, et al. Disease-Modifying drugs for multiple sclerosis in pregnancy; A systematic review. Neurology. 2012;79:1130-1135.
42. Thiel S, Langer-Gould A, Rockhoff M, et al. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis—A prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler. 2016;22:801-809
43. Hellwig K, Rockhoff M, Herbstritt S, et al. Exclusive Breastfeeding and the Effect on Postpartum Multiple Sclerosis Relapses. JAMA Neurol. 2015;72(10):1132–8.
44. Almas S, Vance J, Baker T, Hale T. Management of Multiple Sclerosis in the Breastfeeding Mother. Mult Scler Int. 2016;2016:6527458.
45. Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis – Clinical outcome and prognostic factors. Mult Scler. 2017;23:1241–1248.
46. Kister I, Spelman T, Alroughani R, et al. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study. J Neurol Neurosurg Psychiatry. 2016;87:1133–1137.
47. Hua LH, Fan TH, Conway D, et al. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler. 2019;25:699-708.
48. Ma X, Wang D, Wu G, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
49. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
50. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatientswithCOVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054-1062.
51. D'Antiga I. Coronavirus and immunosuppressed patients. The facts during the third epidemic. Liver Transp. 2020;26:832-834
52. Mehta P, McCauley DF, Brown M. et al. Correspondence COVID-19: Consider cytokine storm syndromes and. Lancet. 2020;6736:19-20.
53. World Health Organization. Critical preparedness, readiness and response actions for COVID-19, March 2020. www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/critical-preparedness-readiness-and-response-actions-for-covid-19. Revised May 2020. Accessed May 7, 2020.
54. MS International Federation. Global COVID-19 advice for people with MS. www.msif.org/news/2020/02/10/the-coronavirus-and-ms-what-you-need-to-know/. Accessed March 13, 2020.
55. Carra A, Steinberg J, Macias-Islas MA, et al. COVID-19 en pacientes con esclerosis múltiple. Recomendaciones latinoamericanas & Sharing initiative.www.lactrimsweb.org/wp-content/uploads/2020/04/2020_04_09_RECOMENDACIONES-LATAM-EM_COVID-19_FOROLATAM.pdf. Accessed April 2020.
56. Costa-Frossard L, Moreno I, Meca-Lallana V, et al. Documento EMCAM para el manejo de pacientes con esclerosis múltiple durante la pandemia de SARS-CoV-2. Rev Neurol .2020; 20: 329-340.
57. Giovannoni G. Anti-CD20 immunosuppressive disease-modifying-therapies and COVID-19, Mult Scler Relat Disord. 2020; epub ahead of print: doi: 10.1016/j.msard.2020.102135